CLO:
Metabolism, Organic Compounds - pp. 68-87 |
|||||||||||
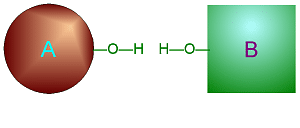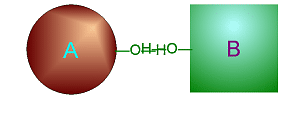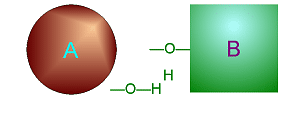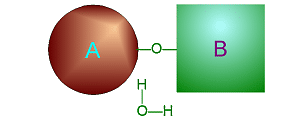
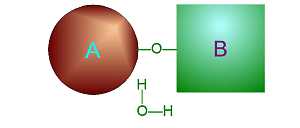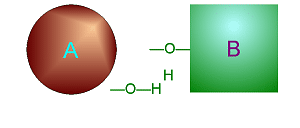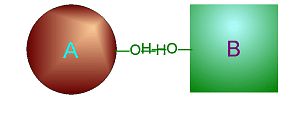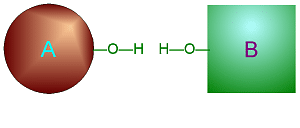
| Metabolism: All chemical reactions done in the body. Catabolic: Breakdown/decomposition of substrate. Release of energy (exergonic) Anabolic: Buildup/synthesis of needed material. Absorption of energy (endergonic). * Energy of of a chemical bond is carried by the electron. If during a covalent chemical reaction a molecule gains an electron it is reduced (negative is even lower). If it loses an electron it is oxidized. This is how energy is transfered metabolically an will be discussed in detail later with ATP synthesis. Organic Compounds and Functional groups As previously mentioned, because carbon has four valence electrons and makes covalent bonds, it can form molecules that are long chains. There are several different kinds of these we will be discussing: hydrocarbons, carbohydrates, lipids, proteins, and DNA. Because of the many important and unique properties of carbon-based molecules, there is a special branch of chemistry devoted just to the study of these molecules. Organic chemistry is the study of compounds containing carbon. Plain hydrocarbons are not found in in living cells. There are usually other groups of atoms attached somewhere on the molecule. There are certain groups of atoms that are frequently attached to the organic molecules we will be studying, and these are called functional groups. These are things like hydroxyl groups which form alcohols, carbonyl groups which form aldehydes or ketones, carboxyl groups which form carboxylic acids, and amino groups which form amines. These groups tend to act the same and have similar properties no matter where on a carbon backbone molecule they’re stuck. Additionally, a molecule may have more than one functional group and/or more than one type of functional group attached. For example, amino acids, which have both both an amino group and a carboxyl group attached. To synthesize polymers (carbohydrates , lipids , and proteins) entails dehydration synthesis and decomposition , hydrolysis
|